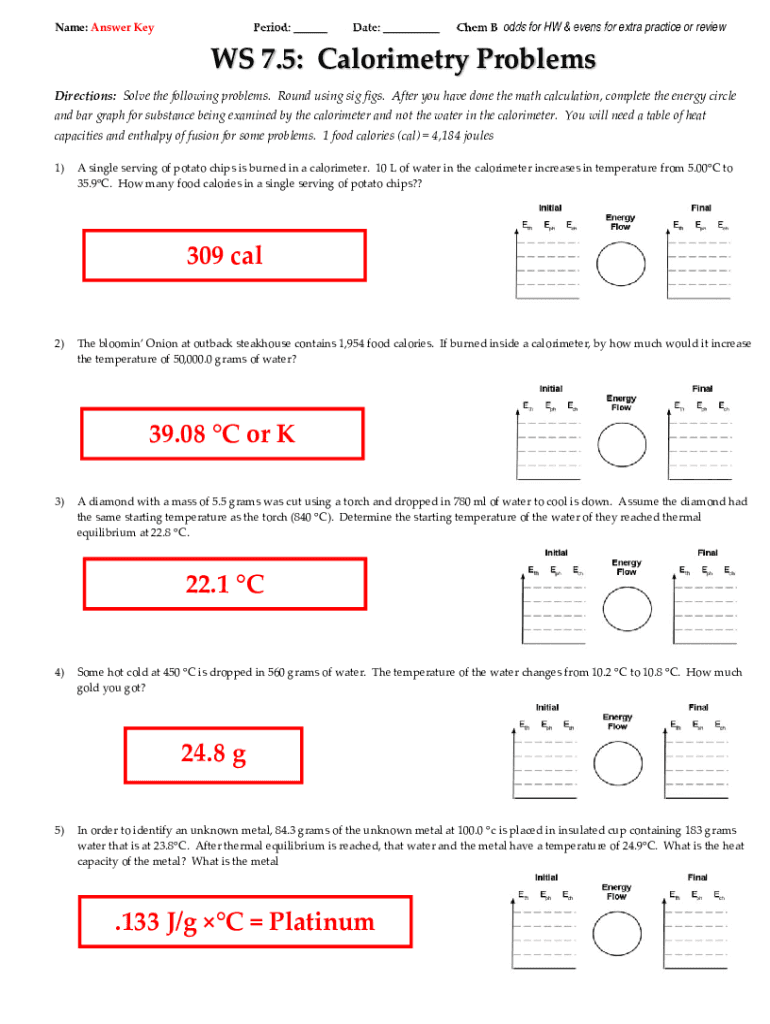
Calorimetry Problems Worksheet Answer Key . How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calorimetry practice problems 1. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added?
from www.pdffiller.com
If the heat capacity of the. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. calorimetry practice problems 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c.
Fillable Online UNIT 7 Assignment 5 Calorimetry Problems Answer
Calorimetry Problems Worksheet Answer Key 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. If the heat capacity of the. calorimetry practice problems 1. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
From printabledermatozekz.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Problems Worksheet Answer Key 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity. Calorimetry Problems Worksheet Answer Key.
From tomdunnacademy.org
Unlock the Answers Calorimetry Worksheet Answer Key Revealed Calorimetry Problems Worksheet Answer Key 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calorimetry practice problems 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. 1) if a gold ring with a mass of 5.5 g changes temperature. Calorimetry Problems Worksheet Answer Key.
From materialdbtracy.z13.web.core.windows.net
Calorimetry Pogil Worksheet Answer Key Calorimetry Problems Worksheet Answer Key in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. If the heat capacity of the. 1). Calorimetry Problems Worksheet Answer Key.
From excelkayra.us
Calorimetry Problems Worksheet Answers Kayra Excel Calorimetry Problems Worksheet Answer Key 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? If the heat capacity of the. How much energy is needed. Calorimetry Problems Worksheet Answer Key.
From www.englishworksheet.my.id
Calorimetry Worksheet Answer Key Englishworksheet.my.id Calorimetry Problems Worksheet Answer Key What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? calorimetry practice problems 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. 1) if. Calorimetry Problems Worksheet Answer Key.
From learningbasilejem.z22.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Problems Worksheet Answer Key calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? calorimetry practice problems 1. How much energy is needed to change the temperature of 50.0 g of. Calorimetry Problems Worksheet Answer Key.
From www.onlineworksheet.my.id
Calorimetry Worksheet Answer Key Onlineworksheet.my.id Calorimetry Problems Worksheet Answer Key calorimetry practice problems 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of. Calorimetry Problems Worksheet Answer Key.
From www.englishworksheet.my.id
Calorimetry Worksheet Answer Key Englishworksheet.my.id Calorimetry Problems Worksheet Answer Key 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems 1. . Calorimetry Problems Worksheet Answer Key.
From printabledermatozekz.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Problems Worksheet Answer Key If the heat capacity of the. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How much energy is needed to change the temperature of 50.0 g of water. Calorimetry Problems Worksheet Answer Key.
From imsyaf.com
Calorimetry Worksheet Answer Key Calorimetry Problems Worksheet Answer Key calorimetry practice problems 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? calorimetry. Calorimetry Problems Worksheet Answer Key.
From studylib.net
Calorimetry practice Calorimetry Problems Worksheet Answer Key How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. If the. Calorimetry Problems Worksheet Answer Key.
From excelkayra.us
Calorimetry Worksheet Answer Key Kayra Excel Calorimetry Problems Worksheet Answer Key If the heat capacity of the. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? calorimetry practice problems 1.. Calorimetry Problems Worksheet Answer Key.
From learninglibrarysteiner.z21.web.core.windows.net
Heat Practice Problems Worksheets Calorimetry Problems Worksheet Answer Key How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. If the heat capacity of the.. Calorimetry Problems Worksheet Answer Key.
From learningarrigskap7f.z22.web.core.windows.net
Heat And Temperature Worksheet With Answers Calorimetry Problems Worksheet Answer Key when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go over the main. Calorimetry Problems Worksheet Answer Key.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Problems Worksheet Answer Key calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1) if a gold ring with a. Calorimetry Problems Worksheet Answer Key.
From www.scribd.com
Answers TC Calorimetry Practice Questions Continuum Mechanics Calorimetry Problems Worksheet Answer Key calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. What is the specific heat of aluminum if the. Calorimetry Problems Worksheet Answer Key.
From studyschoolburman.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Calorimetry Problems Worksheet Answer Key calorimetry practice problems 1. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oc when 207 j of heat is added? 1) if. Calorimetry Problems Worksheet Answer Key.
From www.pinterest.com
Calorimetry Worksheet Answer Key Calorimetry Worksheet Calorie Calorimetry Problems Worksheet Answer Key calorimetry practice problems specific heat for water = 4.184 j/g0c q=mxatxc 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. 1) if a gold ring with a mass of 5.5 g changes temperature. Calorimetry Problems Worksheet Answer Key.